Background introduction
It was discussed that glutathione (GSH) can prevent protein from oxidative damage and maintain immune function.
Butylated hydroxytoluene (BHT) is commonly used as an antioxidant in foods such as cooking oil, canned food, baby candy and meat products.
BHT is considered a potential tumor promoter, carcinogen and endocrine disruptor, and excessive intake can harm human health.
BHT is absorbed into the blood through the intestine and binds to serum albumin, which may change the structure of serum albumin (BSA) and even affect its normal physiological function.
Glutathione can inhibit the changes of BHT on the secondary structure and amino acid microenvironment of BSA, and stabilize the structure of BSA to some extent.
Article highlights
- The effect of glutathione (GSH) on the binding of butylated hydroxytoluene (BHT) to bovine serum albumin (BSA) investigated.
- It provides reference information for understanding the effect of BHT on protein structure and function;
- It found that GSH could inhibit the changes of BHT’s secondary structure and amino acid microenvironment of BSA, but could not reduce the antioxidant activity of BHT.
Content introduction
1, Experimental part
1,1 Main instruments and reagents
1,2 Experimental methods
2, Results and discussion
2,1 fluorescence quenching method
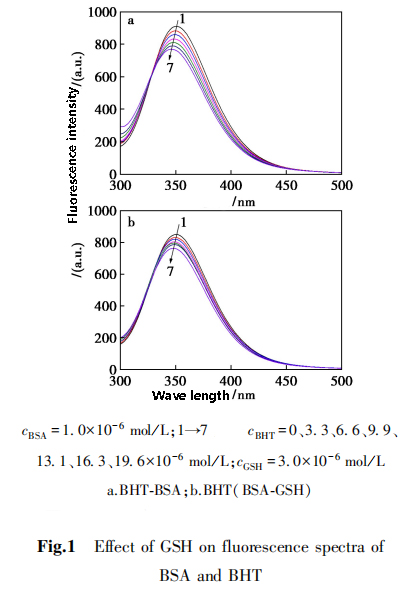
Fluorescence quenching method used to study the interaction mechanism between BHT and BSA.
The quenching of BSA fluorescence spectra by BHT and the effect of glutathione shown.
2,2 Chemical mechanism
In general, the larger the Kb value, the stronger the binding affinity, which may mean that BHT will not rapidly metabolized and will accumulate in the body, and the inhibitory effect of glutathione on the binding of BHT and BSA may speed up the metabolism of BHT, so that it will not be too much accumulation.
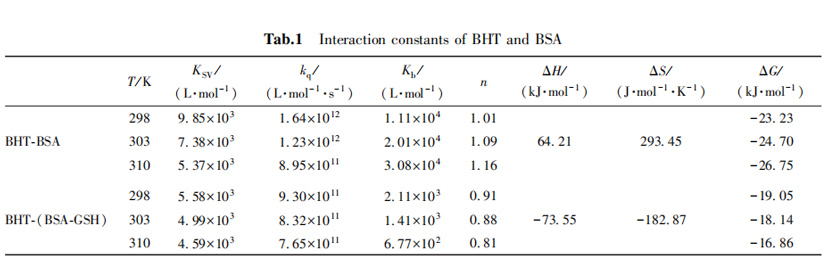
2,3 Thermodynamic parameters and types of forces
2,4 Energy transfer and action distance
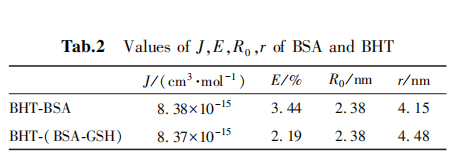
Based on the Forster non-radiative energy transfer theory, the spectral overlap integral J, energy transfer efficiency E, critical distance R0 and action distance r between BSA and BHT can calculated as shown in Table 2.
The change of 2,5 BHT on the conformation of BSA and the effect of glutathione
2,5,1 Ultraviolet spectrum
Ultraviolet spectroscopy can used to study the effect of BHT on the conformation of BSA.
The two characteristic absorption peaks of BSA at 210 nm and 278 nm reflect the conformation of BSA peptide chain and the polarity of microenvironment, respectively.
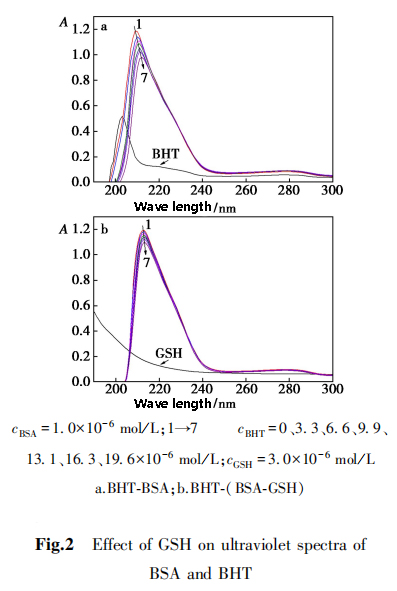
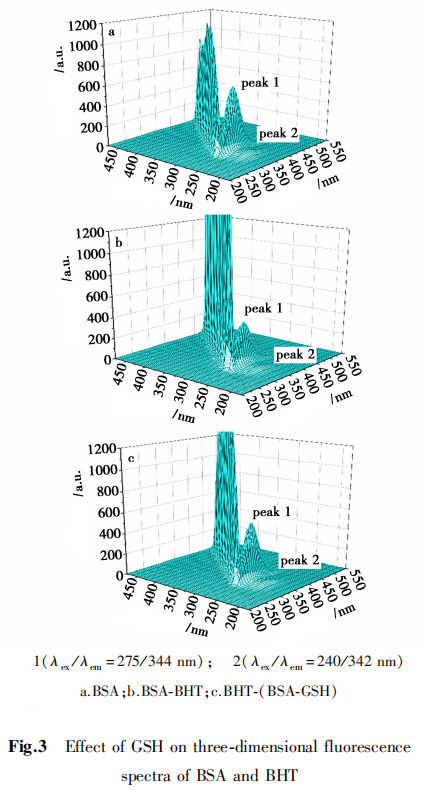
2,5,2 three-dimensional fluorescence spectrum
Three-dimensional fluorescence spectra can used to further study the conformational changes of BHT on BSA.
BSA exhibited two characteristic fluorescence peaks, peak 1 and peak 2 reflecting the characteristics of amino acid residues and the backbone structure of the polypeptide chain, respectively, as shown in Figure 3.
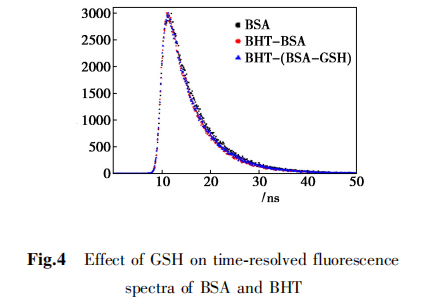
2,6 Time-resolved fluorescence spectra
Time-resolved fluorescence spectroscopy used to determine the effect of BHT on the fluorescence lifetime of BSA, as shown in Figure 4.
Competition at 2,7 sites
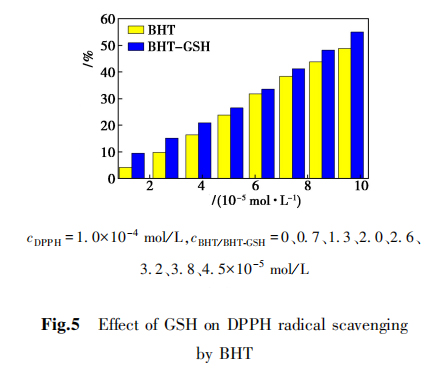
2,8 The BHT clears the DPPH
The DPPH free radical scavenging capacity of BHT can reflect the antioxidant capacity of BHT, as shown in Figure 5.
3 Conclusion
Glutathione can affect the binding of BHT and BSA,
mainly by weakening the binding affinity of BHT and BSA, and changing the binding force of the two.
The inhibition of glutathione on the binding process of BHT and BSA may reduce the accumulation of BHT in blood,
shorten the half-life of BHT in plasma, and reduce its threat to human health.
The ultraviolet absorption spectrum, three-dimensional fluorescence spectrum and time-resolved fluorescence spectrum respectively verified that glutathione could inhibit the changes of BHT on the secondary structure and amino acid microenvironment of BSA, and stabilize the structure of BSA to a certain extent.
In conclusion, glutathione may be an effective protective agent to reduce the toxicity of BHT to transport proteins.