Garbage cleaning master: Using NMN to promote mitochondrial autophagy, breaking the chain of aging
The physical function of the human body gradually declines, which is a natural process that everyone needs to face. However, the mechanism of aging and how to delay the rate of aging has been a hot topic for scientists to study.
On January 15, 2024, a study published by a team from Harbin Medical University revealed a close link between aging and the tumor microenvironment, mitochondrial homeostasis, and the immune system.

The development of T cell senescence is a common feature of the tumor microenvironment
It is well known that T cells are an important part of the immune system and can help the body fight disease. However, the researchers found that T cells in the tumor microenvironment showed senescence, which could be a major barrier to successful tumor growth.
The researchers did an experiment with mice, implanted the melanoma cell line B16F0 into the mice, and after the tumor grew to a certain size, isolated T cells from the spleen for analysis.
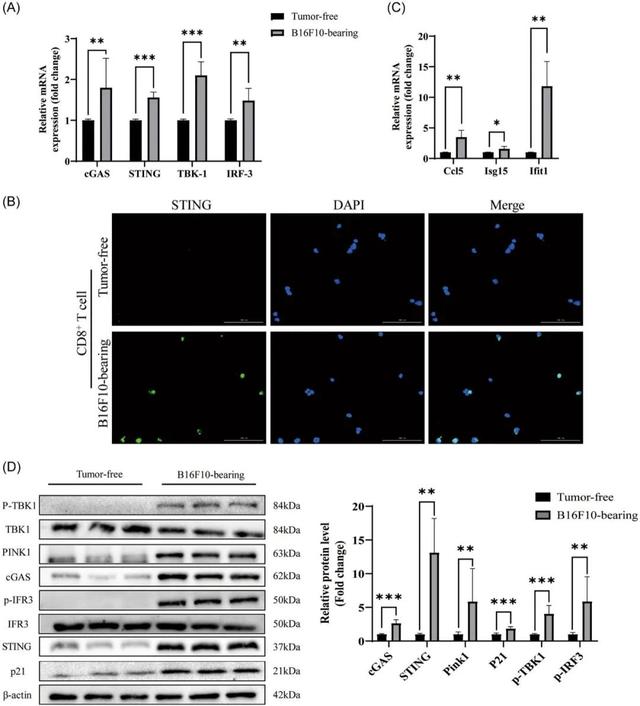
They found that some of the CD8+T cells in these mice showed signs of aging, such as a decrease in the proportion of CD4+ and CD8+T cells, and an increase in the expression of genes encoding certain inflammatory factors.
In addition, the researchers found increased mRNA expression of genes encoding IL-1β, IL-6, TNF-α, and difn-γ in the CD8+T cells of these tumor-bearing mice compared to mice without tumors, suggesting that these inflammatory factors play an important role in aging T cells.
These inflammatory factors may also trigger responses from other immune cells that affect the function of the immune system as a whole.
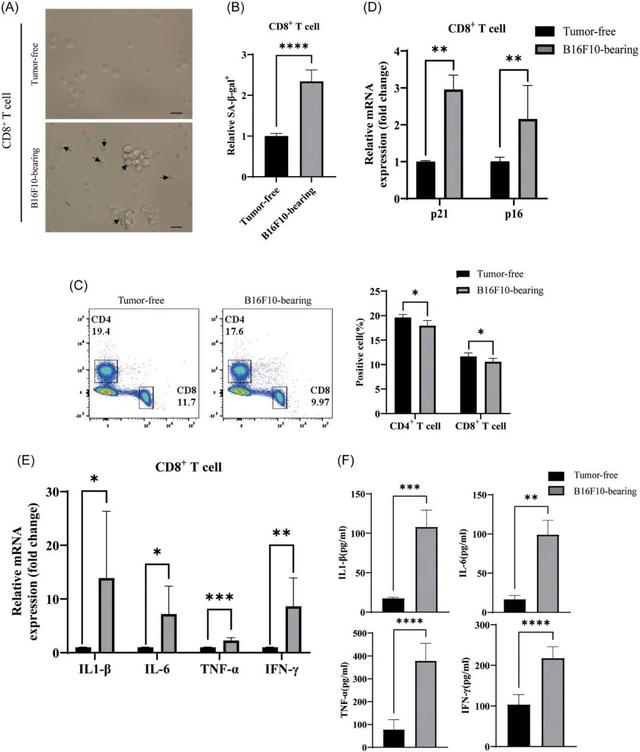
Effects of aging CD8 +T cell disorder on mitochondria
In the tumor microenvironment, the aging of T cells not only affects the function of the immune system, but also provides a favorable environment for tumor growth. To further investigate the mechanism of CD8 +T cell aging, the researchers explored its relationship with mitochondrial function and NAD+ levels.
It has been found that cell senescence is closely related to mitochondrial damage. Mitochondria are the energy factories of the cell, responsible for the production of ATP, and are also the main source of ROS.
In senescent CD8 +T cells, they observed depolarization of mitochondrial membrane potential, increased ROS levels, and decreased ATP levels, suggesting that mitochondrial function was severely impaired.
In addition, they found that both NAD+ levels and NAD+/NADH ratios in senescent CD8 +T cells were lower than in normal cells.
NAD+ plays a key role in many biological processes, including energy metabolism, DNA repair, and cell signal transduction. A decrease in NAD+ levels may affect these processes, thereby accelerating the aging of cells.
Importantly, free cytoplasmic mtDNA detected from the damaged mitochondria. mtDNA is the genome in mitochondria, and its release into the cytoplasm can trigger an inflammatory response. The findings suggest that the release of mtDNA may be a trigger for T-cell inflammation and aging.
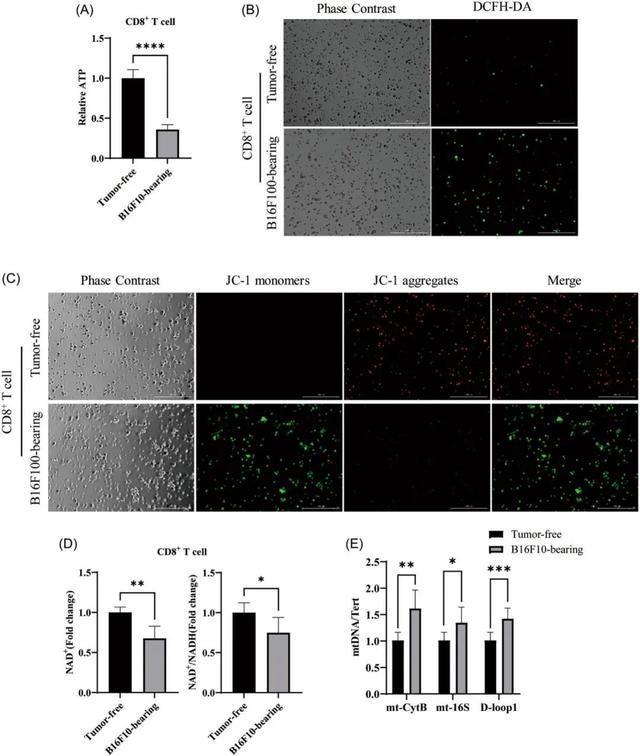
The STING pathway is activated in senescent CD8+T cells
Cellular aging causes a number of changes, one of which is that certain pathways in CD8+T cells are activated.
In previous studies, scientists found that senescent cells release damaged DNA into the cytoplasm, which activates a protein-mediated pathway called STING, which in turn triggers the production of certain factors that promote the cell’s aging.
In this study, the researchers further tested whether the cGAS-STING pathway activated in senescent CD8+T cells.
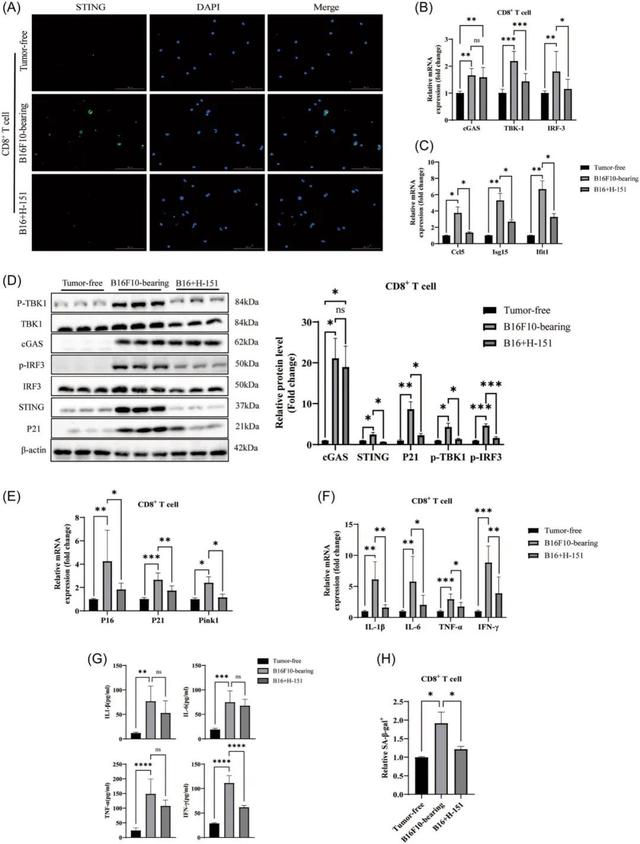
The results showed that mRNA levels of the proteins cGAS, STING, TBK1 and IRF3 were all increased in senescent CD8+T cells.
In addition, STING expression found to up-regulated and clustered around the nucleus. In addition to the activation of cGAS and STING, downstream TBK1 and IRF3 proteins also activated in the form of increased phosphorylation.
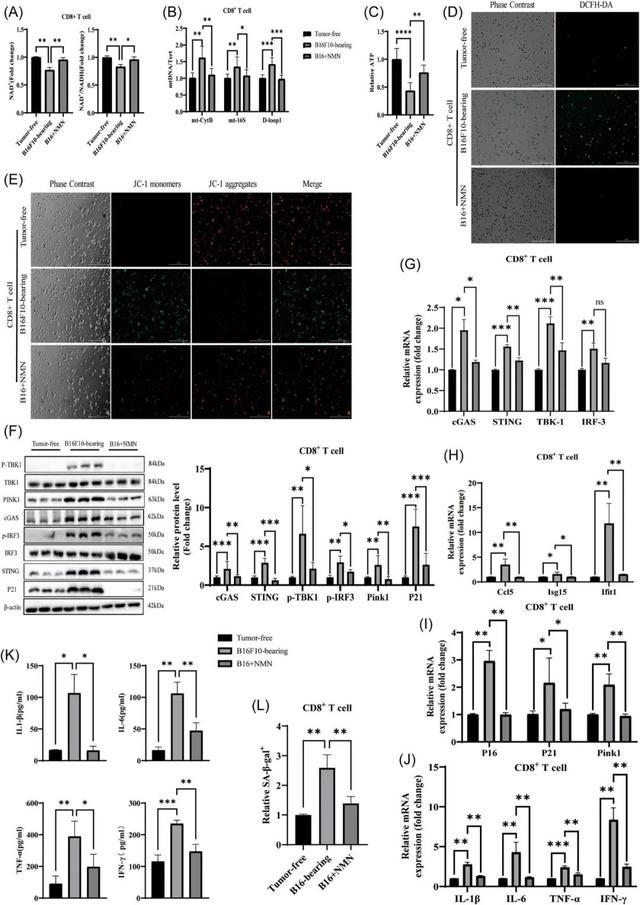
The activation of cGAS-STING can be inhibited to reduce cell senescence
To determine definitively whether the tumor microenvironment influences the aging of CD8+T cells through the cGAS‐STING pathway. The researchers used a specific STING inhibitor, H‐151 (2 μM,MCE, #HY‐12693), to investigate the effects of CD8+T cell senescence.
The study showed that STING enrichment reduced in H-151 treated cells, and the treatment significantly reduced the expression of aging signaling mediators.
The protein and mRNA level results showed that the cGAS‐STING pathway activated during aging, but cGAS remained highly expressed after the use of inhibitors.
STING and its downstream molecules decreased significantly. In addition, after the use of H-151, the signaling medium of aging significantly reduced.
In further experiments, the researchers found that the percentage of stained cells in cells treated with H-151 reduced compared to senescent CD8+T cells. This suggests that activated STING plays a key role in cellular senescence.
NMN improves mitochondrial DNA and alleviates STING aging
Based on these experimental results, the researchers also investigated whether increasing intracellular NAD+ levels with NMN can prevent aging.
The results showed that after 3 days of NMN supplementation (100 μM, Absin, #abs47048054), the level of NAD+ in senescent CD8 +T cells recovered, and the ratio of NAD+ /NADH up-regulated.
Consistent with previous findings, mtDNA significantly enriched in the cytoplasm of senescent cells. However, NMN supplementation(NMN supplier) decreased cytoplasmic mtDNA content.
In addition, NMN reduced ROS (reactive oxygen species) levels and mitochondrial membrane potential in these cells, and increased intracellular ATP.
The researchers also found that after pretreating senescent CD8+T cells with NMN, activation of the cGAS-STING pathway reduced.
This suggests that NMN can act like STING inhibitors. In addition, NMN supplementation can significantly reduce the secretion of inflammatory factors, further proving that NMN can improve the aging state of cells.
Taken together, these experimental results clearly show that by inhibiting the STING pathway, NMN can significantly improve the aging state of T cells and reduce the secretion of inflammatory factors associated with aging.
The role of NMN in preventing neuroinflammation and aging in mice
Current in vitro studies have shown that activation of the cGAS‐STING pathway is a checkpoint controlling T cell senescence and function mediated by tumor cells.
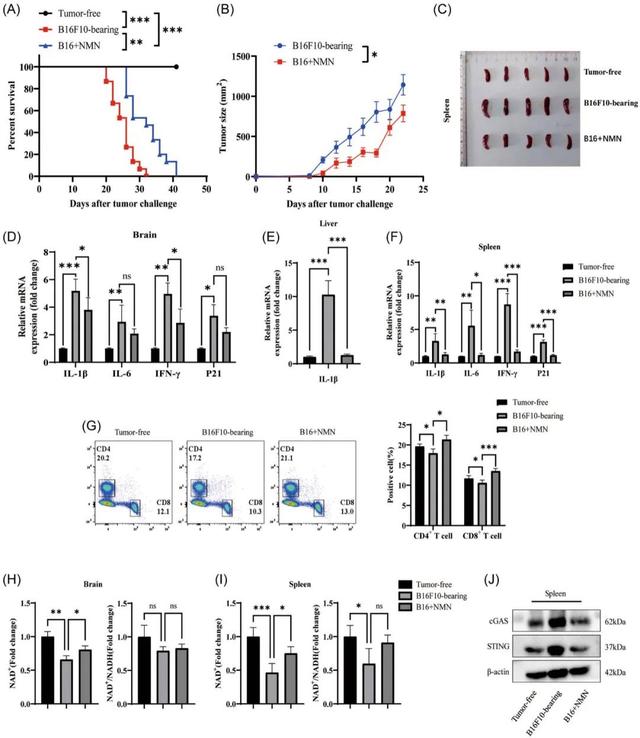
So the researchers conducted an experiment in which mice were injected intraperitoneally with NMN for 21 days. The results showed that tumor growth suppressed in the NMN-treated mice and they survived longer.
In addition, the researchers compared spleen size and organ molecular changes in different groups of mice. Results showed that NMN treatment mitigated tumor-induced splenic enlargement and inhibited the expression of inflammatory factors such as IL-1β, IL-6, IFN-γ, and P21.
At the same time, NMN treatment also increased the content of CD4+ and CD8+T cells in the spleen, and increased the NAD+/NADH ratio.
Based on these data, it appears that NMN therapy through the STING pathway has the potential to increase anti-tumor activity, reduce neuroinflammation, improve aging conditions, and prolong survival in mice.
In summary, the experiment demonstrated the relationship between the cGAS-STING pathway and inflammation and aging phenotypes. Damaged mitochondria release substances that activate the cGAS-STING pathway, leading to inflammation and aging.
Supplementing with NAD+, also known as NMN, can help clear damaged mitochondria, thereby inhibiting inflammation and aging. Therefore, maintaining mitochondrial quality may help prevent aging and age-related diseases.
References:
[1]Ye B, Pei Y, Wang L, Meng D, Zhang Y, Zou S, Li H, Liu J, Xie Z, Tian C, Jiang Y, Qiao Y, Gao X, Zhang Y, Ma N. NAD+ supplementation prevents STING-induced senescence in CD8+ T cells by improving mitochondrial homeostasis. J Cell Biochem. 2024 Jan 15.
